Drilling for Soil Chemistry in Geotech Reports, Part 2
What Can Drillers Get out of All Those Data Tables?

We started our look into the value of geotechnical reports for drillers in our last installment. That column talked about the basics of geotechnical reports, as well as what bore logs can tell us. This month, we get into data tables, keywords searches and what these reports can tell us about hydrocarbons.
First, let’s make sense of those data tables.
Data Tables
Potentially the most useful, but usually the most frustrating, portion of any geotech report is the data section. You typically find it toward the end of a geotech report and it appears as a series of tables and charts. These tables show all the lab-testing data, including raw values for salts, hardness, hydrocarbons and the like.
We generally test site chemistry in one of three ways. The first and most common way is via Toxicity Characteristic Leaching Procedure (TCLP) of a dry soil. TCLP involves acid digestion of soil with a measurement of acid-solubilized ions. Labs measure the ions by Inductively Coupled Plasma (ICP), a test that gives percentages of various elements in a liquid medium. We cannot realistically gain any insights into the ability of soil salts to have an impact on our slurries using this acid digestion preparatory method, as concentrated acid does not exist in nature. However, acid-digestion does give theoretical maximum content figures for particular elements and it is important to people who need to know about trace heavy metals like lead, cadmium and mercury in drinking water. Acid will leach out some ions, like aluminum, that simply will not be soluble in normal groundwater or in a drilling fluid. This is the least useful ICP-based prep-method that we will see.
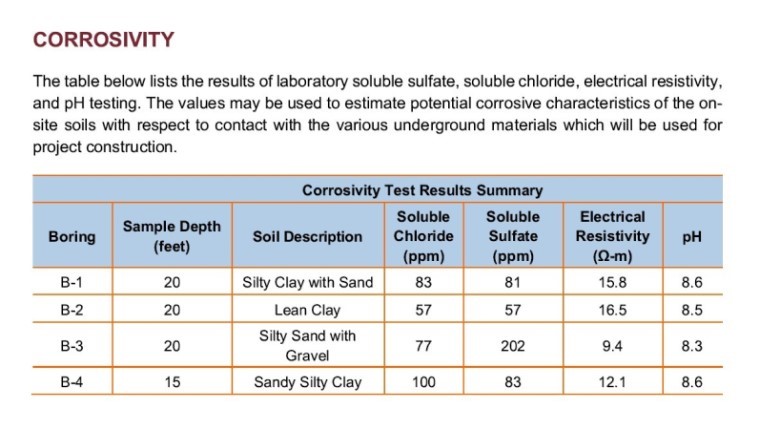
The second type of test involves a leaching of dry soil using simple, neutral pH water in place of an acid. The results this produces give us a reasonable idea of true soil chemistry and are often part of a corrosivity testing method. The soluble salts here mostly include chlorides, sulfates, nitrates, and carbonates of sodium, magnesium and calcium. You rarely see values for other elements high enough to have an impact on our slurry. In the report, note any particular ions with values above 100 ppm (mg/L). Below this, the ion is not impactful to viscosity or filtrate loss. Notes about the leaching process may mention a ratio, like 1:2 or 1:10. This is the weight ratio of dry soil to water used to make the leach. Water leaching often produces concentrations of salts lower than what we might find in groundwater; if both water leach and groundwater data are present in a report, the water leach usually shows less solubilized sodium than what the groundwater shows. This is due primarily to the amount of time involved in the leaching process. Water leaches often use as little as 24-hours, whereas groundwater may leach from soil over years or even centuries.
The third type of ICP data that we may find is the best: direct testing of groundwater or mix-water (municipal, lake, river, etc.). Liquid water testing gives the most accurate measurement of what salts are present underground or in a mix-water. Again, expect to see higher values for chlorides, sulfate, nitrates, carbonates and the associated cations such as sodium, calcium, magnesium and occasionally iron. Ignore ionic concentrations below 100 ppm (mg/L).
Search for Keywords
Often, a basic geotech report gets distributed as a PDF file. One simple trick for longer reports involves for certain keywords that pertain to what most affects a drilling fluid’s performance. Some of these keywords:
- Salt, electrical conductivity, EC, total-dissolved solids or TDS: These terms tell us about how much salt is present in soils and waters. These values are an indirect measurement of the sum of all the ICP-measured ions. EC values above 3000 μS/cm will impact a bentonite fluid badly, but many polymers can only handle a few hundred microSiemens of electrical conductivity before losing significant viscosity. Salt will hurt synthetic polymers more than bentonite. Remember that many high viscosity bentonite products contain synthetic extending polymers. Biopolymers are more resistant than the synthetic types.
- A ‘corrosivity’ or ‘resistivity’ evaluation: Also tells you everything you need to know about salt content in the soil. Electrical resistivity, measured in ohms/distance, is the inverse of electrical conductivity. Ohms uses the Greek letter omega (Ω). For bentonites, be concerned when resistivity is lower than 3.33 Ω/m (3000 μS/cm in terms of EC). For synthetic polymers, this increases to 12.5 Ω/m (800 μS/cm). Remember to watch unit conversions.
- One can also search for individual ions, including chloride, sulfate, calcium, magnesium, sodium and nitrates. Typically, those are the major anions and cations of concern. While any level of lead might be bad to drink, we rarely find soluble levels high enough to have an impact on a bentonite or polymer drilling fluid. (The ions we are most concerned with, however, are both more common and, unfortunately, less likely to show up in a geotech report.) You can add up the ppm of all the soluble ions to get a sense of what the EC might be.
- Total hardness (TH): You may see values given in mL/kg or ppm to describe the total content of calcium, magnesium and other multivalent cations. Hardness is bad news for synthetic polymers, as it inhibits swelling even at low concentrations. Bentonite is more tolerant of high hardness, but values above about 300 ppm should still be treated out with soda ash, caustic soda or bicarbonate.
- Parts per million (ppm) values: The units most often used in geotech reports to describe salt content. You may also see “mL/L” or “mg/kg.” Both are equal to ppm but represent soluble content versus total dry-solids weight content. Anything with mL, ml or milliliters describes a liquid or liquid-leach of soil. I always prefer this liquid data over mg/kg, since the solid content of an element does not tell us whether that element is soluble or not. For example, a value of 2000 mg/kg aluminum would be terrible if solubilized into our mix water, but since aluminum is mostly insoluble in nature, we can safely ignore values shown in mg/kg. Many heavy metals like lead, mercury, barium and the like are only be soluble in low pH situations (below 5, generally).
Unfortunately, searching for “pH” can prove frustrating and difficult as so many words have this letter combination. Even when using quotation marks to limit the search to only “pH,” I find the search fails in most cases.
This table shows what you might find on a typical ICP water analysis. Ion concentrations high enough to affect mud appear here in red.
|
Element |
Concentration in ppm |
|
Ag |
Below detectable limits |
|
Al |
1.240 ppm |
|
As |
Below detectable limits |
|
B |
2.159 ppm |
|
Ba |
0.0139 ppm |
|
Ca |
144.5 ppm |
|
Cd |
Below detectable limits |
|
Cl |
886.2 ppm |
|
Cr |
0.1221 ppm |
|
Cu |
0.0049 ppm |
|
Fe |
0.0996 ppm |
|
Hg |
Below detectable limits |
|
K |
180.7 ppm |
|
Mg |
339.5 ppm |
|
Mn |
0.0137 ppm |
|
Mo |
Below detectable limits |
|
N |
Below detectable limits |
|
Na |
1,934 ppm |
|
Ni |
Below detectable limits |
|
P |
0.0635 ppm |
|
Pb |
0.0038 ppm |
|
S |
445.6 ppm |
|
Sb |
Below detectable limits |
|
Se |
0.0086 ppm |
|
Ti |
Below detectable limits |
|
Zn |
0.0240 ppm |
|
Zr |
0.0014 ppm |
Hydrocarbons
Geotechnical reports often show the concentrations of hydrocarbons. A report may list gasoline range organics (GRO) and diesel range organics (DRO), along with various benzene-based aromatic hydrocarbons (BTEX) and volatile compounds. Some reports list pesticides, herbicides and fungicides as well — all of which have long, confusing names containing numbers. The good news? You should find most of these hydrocarbons at concentrations to affect a bentonite or polymer slurry. A few, which have strongly polar groups, could act as surfactants and generally wet clay more effectively. Overall, the DRO and GRO organics are hydrophobic and literally hate water, clay or any other surface that absorbs water. On a drill site with high organics, we may often see a sheen or oil slick on the surface of water or our slurry. Polymers and bentonite typically hydrate just fine in the water that sits under the slick. A value over a few thousand ppm might create have an impact by encapsulation, but not if the pH is seven or above. If in doubt, a bit of drilling surfactant can help emulsify the oils floating on the surface of your mix tank. You might want to have a surfactant on hand if the geotech report says anything about oils or fuels in the soil.
The last bit of advice I offer is to ask for more data if you have concerns or doubts. Some of this water-based testing is quite cheap and fast to perform. Often, a customer or engineer will willingly add it to the work. Suggest that they conduct TDS and hardness testing next time. Some of this testing can also be done on-site with a $200 piece of equipment called a conductivity meter and a few pH test strips. Most drilling fluids suppliers also now have special fluids designed specifically for salty and brackish drilling. The cost is inexpensive compared to the cost of keeping a crew onsite for extra days to fight with a stuck bit downhole.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




